Abstract
Background. Imatinib, the first BCR/ABL kinase inhibitor, has drastically changed the chronic myeloid leukemia (CML) scenario improving the rate of responses and long-term outcome. The aim of our analysis was to assess, after a median follow-up of 10.2 years (range 5.8-14.8), the long-term efficacy and safety of imatinib treatment in a monocentric cohort of CML patients treated outside of clinical trials.
Methods. We retrospectively analyzed a series of 458 CML patients treated with imatinib frontline or after interferon (IFN) failure at a single center between 2000 and 2016. Long-term analysis included overall survival (OS), progression-free survival (PFS), event-free survival (EFS), response to treatment and adverse events.
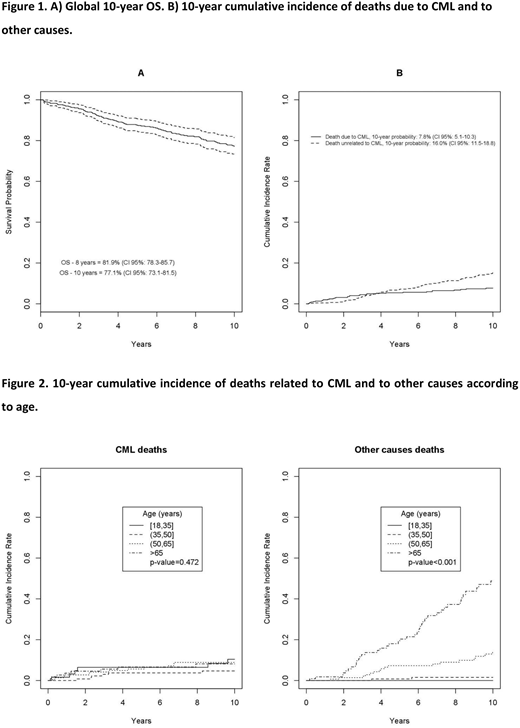
Results. The median age was 55.1 years (range 18.8-91.2). Seventy-one % of patients received imatinib frontline and 29% after IFN failure. Median time of prior IFN treatment was 5.6 years (range 2.4-8.9); the 10-year OS of patients treated with imatinib after IFN and with imatinib frontline was 75.3% (CI 95%: 66.9-81.9) and 77.8% (CI 95%: 72.2-82.5) (p=0.468), respectively. The 10-year-OS of the whole cohort was 77.1% (CI 95%: 73.1-81.5); the 10-year probability of dying due to CML and of other causes was 7.8% (CI 95%: 5.1-10.3) and 16% (CI 95%: 11.5-18.8), respectively (Figure 1). When patients were stratified into 4 groups according to age (18-35, >35-<50, >50-<65 and >65 years), no difference was found in the 10-year OS considering only the CML-related deaths (p=0.472), whereas a difference was observed when all other causes of death were considered (p<0.001) (Figure 2). The b2a2 transcript (detected in 34% of patients) at baseline had a negative impact on the 10-year PFS (p=0.035), but not on the 10-year OS (p=0.151). The Eutos long-term survival (ELTS) better stratified the baseline risk of patients in terms of 10-year OS compared to the Sokal and Hasford scores. The 10-year PFS was 77.1% (CI 95%: 72.6-81), while the 10-year EFS was 55% (CI 95%: 50-59.6), considering as event anything that could lead to treatment discontinuation: intolerance (10.4%), lack of cytogenetic response (17.5%), no major molecular response over time (MMR, 22.6%), lack of molecular response (6.1%), evolution into blast phase (13.7%), death (29.7%). After a median time of 10.2 years, 83 patients (18.1%) achieved as their best molecular response a MMR after a median time of 2.4 years (range IQ 1.36-4.01), 74 patients (16.1%) a MR4.0 after a median time of 4.57 years (range IQ 2.08-7.33), 162 (35.3%) a MR4.5 after a median time of 8.23 years (range IQ 4.14-11.53) and 60 (13.1%) never achieved a MMR during treatment. The achievement of an early molecular response (EMR) at 3-months (BCR/ABL1 <10%) and a MMR at 12-months was associated with a significant advantage in terms of 10-year OS (p=0.006 and p=0.032, respectively) and of 10-year PFS (p=0.004 and p=0.009, respectively). One-hundred and twenty eight patients (27.9%) switched to a second generation inhibitor (2TKI) after a median time of treatment of 3.48 years (range IQ 1.47-5.89); 23 patients (17.9%) were intolerant, while 105 (82.1%) were resistant. Among resistant patients, 81 (77.1%) never achieved a MMR during imatinib, while 24 who had achieved at least a MMR lost it after a median of 14 months (range 6-22). Furthermore, 11 patients (10.4%) experienced a blast phase evolution. The 5-year OS after starting a 2TKI was 85.2% (CI 95%: 73.5-94.3). We collected also the long-term safety with a particular attention to cardiovascular (CV) events and development of solid tumors. In 27 patients (5.8%), a grade 3 CV event occurred during treatment. Eight (29.6%) experienced a myocardial infarction and 3 patients died, 4 (14.8%) a heart failure that required hospitalization, 4 (14.8%) a stroke with 3 deaths, 5 (18.6%) an atrial fibrillation controlled with specific therapy, 3 (11.1%) a venous thrombotic event that required anticoagulant treatment and 3 (11.1%) a peripheral arterial occlusive event with 1 pulmonary embolism. Thirty-nine patients (8.5%) developed a solid tumor during the course of treatment: the most frequent sites involved were the prostate (20.5%), gastrointestinal (15.3%), bladder (12.8%), lung (10.2%) and breast (10.2%).
Conclusions. A 10-year real-life follow-up of CML patients demonstrates that imatinib maintains efficacy over time and is associated with a low rate of CV events and second neoplasias.
Rizzo:Sapienza University, Rome: Other: Resident in Hematology. Foà:GILEAD: Speakers Bureau; INCYTE: Other: ADVISORY BOARD; AMGEN: Other: ADVISORY BOARD; ABBVIE: Other: ADVISORY BOARD, Speakers Bureau; CELTRION: Other: ADVISORY BOARD; NOVARTIS: Speakers Bureau; JANSSEN: Other: ADVISORY BOARD, Speakers Bureau; CELGENE: Other: ADVISORY BOARD, Speakers Bureau; ROCHE: Other: ADVISORY BOARD, Speakers Bureau. Breccia:Novartis: Honoraria; BMS: Honoraria; Incyte: Honoraria; Pfizer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal